Whilst RCFA can be a powerful tool, organizations may well encounter various problems in its implementation. One widespread obstacle will be the resistance to alter, the place employees might be hesitant to undertake new problem-resolving methodologies or are skeptical from the RCFA process.
(three) If a repeat test is conducted, the same test strategy need to be useful for both the Original and repeat tests, and also the repeat test have to be done with similar product or service that may be reflective in the First sample in terms of sample site along with the phase during the manufacturing process from which it was obtained.
The Oakland Nimitz Freeway was a bridge that collapsed during an earthquake even soon after This system to reinforce the bridge. Distinctive engineers had been asked their tackle the situation. Some didn't blame the program or even the department, like James Rogers who said that in an earthquake There may be “an excellent prospect the Embarcadero would do a similar matter the Nimitz did.
RCFA makes use of numerous analytical tactics which include fault tree Examination, Ishikawa diagrams, plus the 5 Whys strategy to systematically uncover the underlying causes for your failure.
The USP sterility test includes two test methods: (i) immediate inoculation of your culture medium and (ii) membrane filtration. Both methods are made use of equally while in the field, with the selection of which one particular to implement currently being determined by the type of products less than assessment, the need to clear away prospective culture inhibitors with the solution, fees, and equipment sources.
Addition of a fresh relationship, new attachment like a dip tube, and inert fuel purging tube in sterilizing filtration skid, which wasn't Section of the validated system.
Responses or questions on document written content cannot be answered by check here OFR team. Be sure to usually do not supply private data or private information.
A far more in depth investigation will become necessary if a clear lead to will not be evident from the initial critique. This entails analyzing these variables:
Has horizontal deployment been considered? Are there comparable processes in the facility or in sister plants which could possibly be impacted? Can preventive action be instigated in comparable processes and technologies prior to it will become a concern there?
The cookie is ready through the GDPR Cookie Consent plugin and it is used to retail store whether consumer has consented to the usage of cookies. It does not retailer any personalized knowledge.
Title your selection: Name needs to be a lot less than people Decide on website a collection: Not able to load your assortment as a result of an mistake
Checklists can be utilized to assist in identification of such faults (e.g., verification of identification of samples, specifications, reagents, and proper planning of samples) and have the advantage of maintaining regularity in Original assessments. The analyst is liable for initiating and documenting the investigation, and reporting the prevalence for the laboratory supervisor and QA inside of a specified timeframe.
Sterile production is really a important process that needs meticulous interest to depth and adherence to rigorous aseptic techniques.
The laboratory supervisor’s evaluation really should be objective and timely and include things like a review in the supporting documentation in addition to a discussion Using the analyst to confirm the analyst’s knowledge of and overall performance of the correct test technique.
 Jonathan Taylor Thomas Then & Now!
Jonathan Taylor Thomas Then & Now! Ariana Richards Then & Now!
Ariana Richards Then & Now! Anthony Michael Hall Then & Now!
Anthony Michael Hall Then & Now!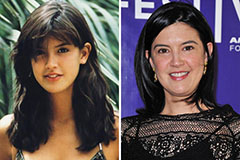 Phoebe Cates Then & Now!
Phoebe Cates Then & Now! Richard Dean Anderson Then & Now!
Richard Dean Anderson Then & Now!